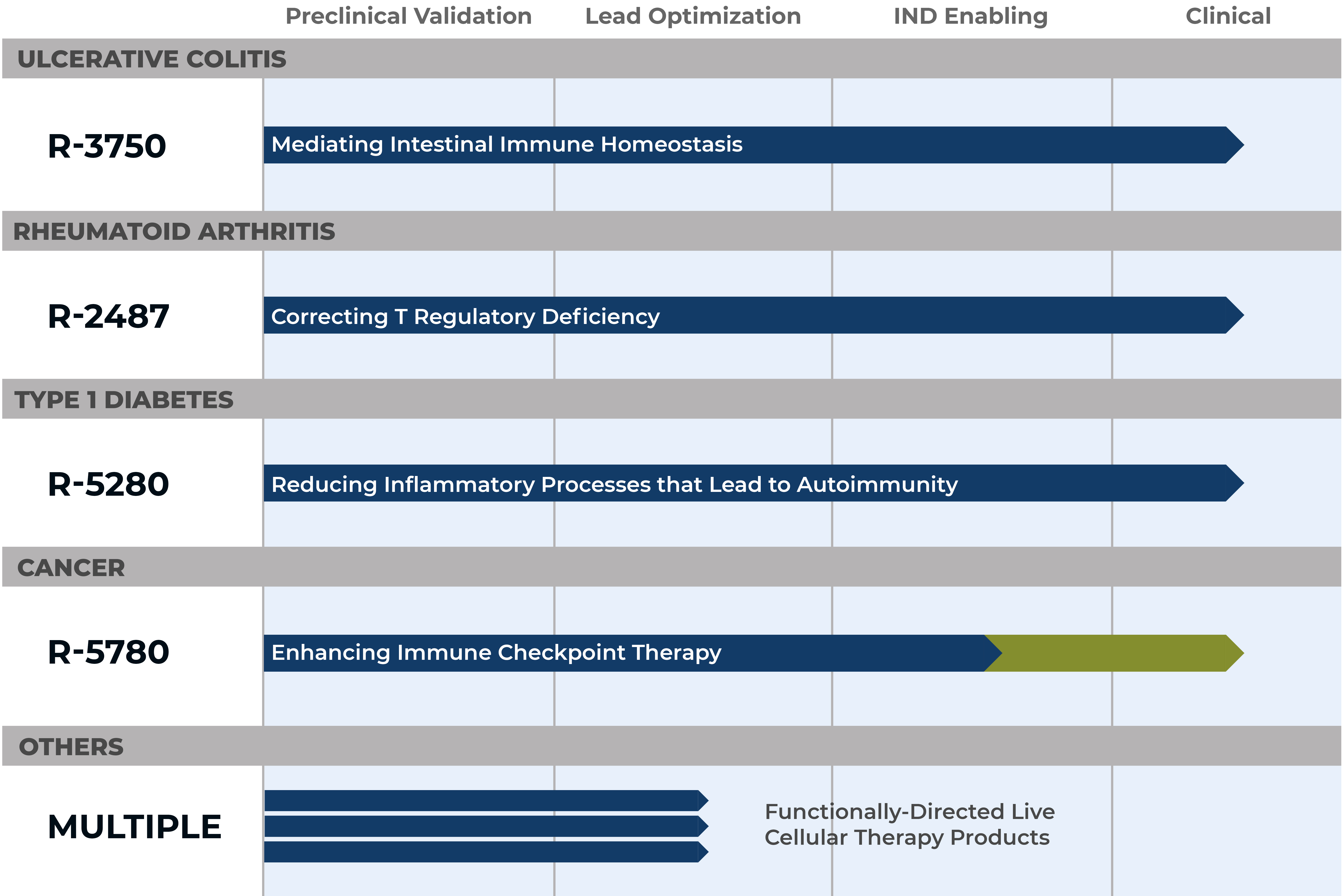
R-3750
R-3750 is an oral immune therapy that has the potential to treat the underlying basis of GI-associated inflammatory disease by inducing immune homeostasis of the intestinal tract. Importantly, R-3750 has the ability to enhance gut barrier integrity, an important mechanism related to successfully treating inflammatory gut disease. R-3750 is based upon targeting of a key microbiome regulatory pathway known to engage immune effectors cells lining the intestinal tract. This program is being developed for the treatment of inflammatory bowel disease (IBD).
Rise Therapeutics is currently running a Phase 1 clinical trial for R-3750 for the treatment of ulcerative colitis. The Phase 1 trial is a single and repeat dose study assessing the safety and tolerability, drug exposure, and clinical activity of R-3750 in patients with mild to moderate ulcerative colitis. The study will enroll up to 36 participants where clinical activity will be evaluated by improvement in ulcerative colitis disease severity and a variety of key biomarker and pharmacodynamic assessments.
If you suffer from ulcerative colitis and want to consider participating in the study, more details can be found at NCT05666960.
R-2487
R-2487 is a targeted oral immune therapy that has the ability to induce T regulatory cell populations. R-2487 has a unique mechanism of action by which it can engage specific immune pathways that regulate tolerance and reset T regulatory deficiencies to reduce specific inflammatory cytokines that contribute to autoimmune disease. This mechanism is particularly important in the context of treating autoimmune disease. R-2487 is being developed for the treatment of rheumatoid arthritis and other autoimmune diseases.
Rise Therapeutics is currently enrolling rheumatoid arthritis patients in a Phase 1 clinical trial for R-2487. The Phase 1 trial encompasses a two-stage enrollment strategy to evaluate both single and repeat dosing to assess the safety, drug exposure, and clinical activity of R-2487 in patients with mild to moderate disease. The study will enroll up to 36 participants where clinical activity will be evaluated by improvement in disease severity and by a select subset of key biomarker and pharmacodynamic assessments.
If you suffer from rheumatoid arthritis and want to consider participating in the study, more details can be found at NCT05961592.
R-5280
R-5280 is an oral immunotherapy focused on controlling immune pathways that lead to autoimmune disease. This product candidate has been selectively designed to engage the immune system to control inflammation by influencing immune cell differentiation and inflammatory cytokine production that contribute to autoimmunity. R-5280 has demonstrated improvement of type 1 diabetes symptoms in previous clinical studies.
Rise Therapeutics has embarked on a double blind, placebo controlled clinical trial to evaluate the safety, efficacy, and biomarker responses of R-5280 in newly diagnosed type 1 diabetes patients. The clinical study will enroll 39 participants to assess 12-week oral administration of R-5280.
If you or a family member suffer from type 1 diabetes, and want to consider participating in the study, more details can be found at NCT06057454.
R-5780
R-5780 is an orally delivered immune oncology drug candidate that engages immune pathways for the treatment of cancer. R-5780 has demonstrated pronounced ability to enhance immune checkpoint activity. This drug candidate builds upon the growing understanding of the mechanisms by which the microbiome can promote efficacy of immune oncology drugs. Rather than building a product around natural commensals where it can be difficult to understand mechanism of action, R-5780 is a targeted drug product that engages selective immune pathways that can drive robust anti-tumor responses.
