Promoting Human Health
and Well Being
Our Science
Innovation
Merging our understanding of immune therapy with cutting-edge discoveries of how the microbiome functions, Rise is developing targeted immunotherapy drugs to treat a broad range of clinical indications.
Immunology
Read more
Microbiome
Read more

Synthetic Biology
Read more
Our Capabilities
PLATFORM
Synthetic Biology Medicine
As a foundational technology, Rise Therapeutics has developed the innovative and proprietary Tripartite X (TPX) drug delivery platform that embraces synthetic biology-based approaches to enable oral delivery of targeted biologic therapy.
INFRASTRUCTURE
Clinical
GMP
Manufacturing
Our clinical GMP drug production capabilities for microbial-based products including Live Biotherapeutic Products (LBPs) enables rapid and efficient translation of innovative bench discoveries towards commercialization.
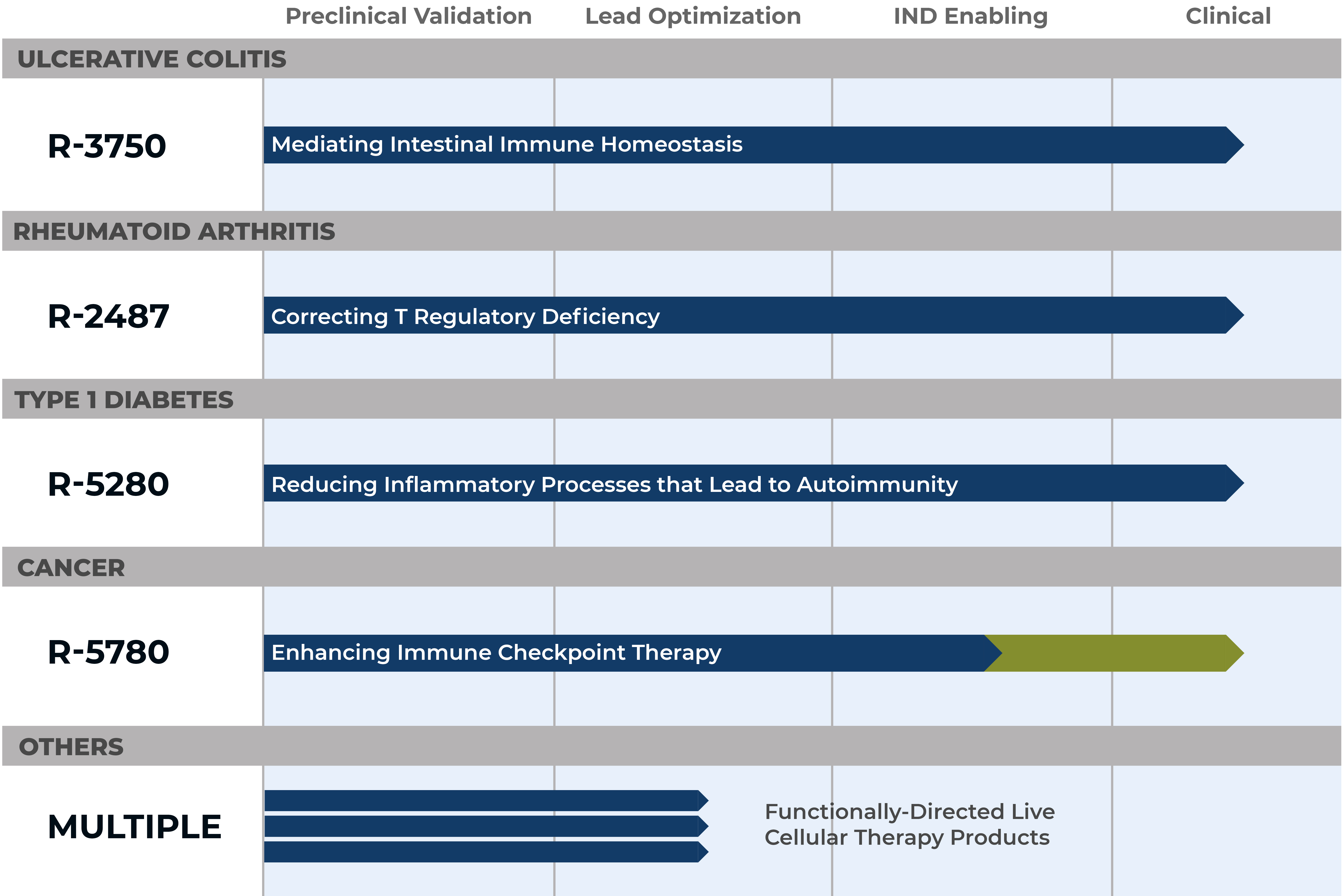
Our Pipeline
At Rise Therapeutics, our product development focus, in-house clinical GMP manufacturing capabilities, and drug development experience enables us to pursue rapid and efficient novel drug development from innovative laboratory discoveries.
Our Team
Interested in working with Us?
Become a Partner
Read more
Join Our Team
Read more





